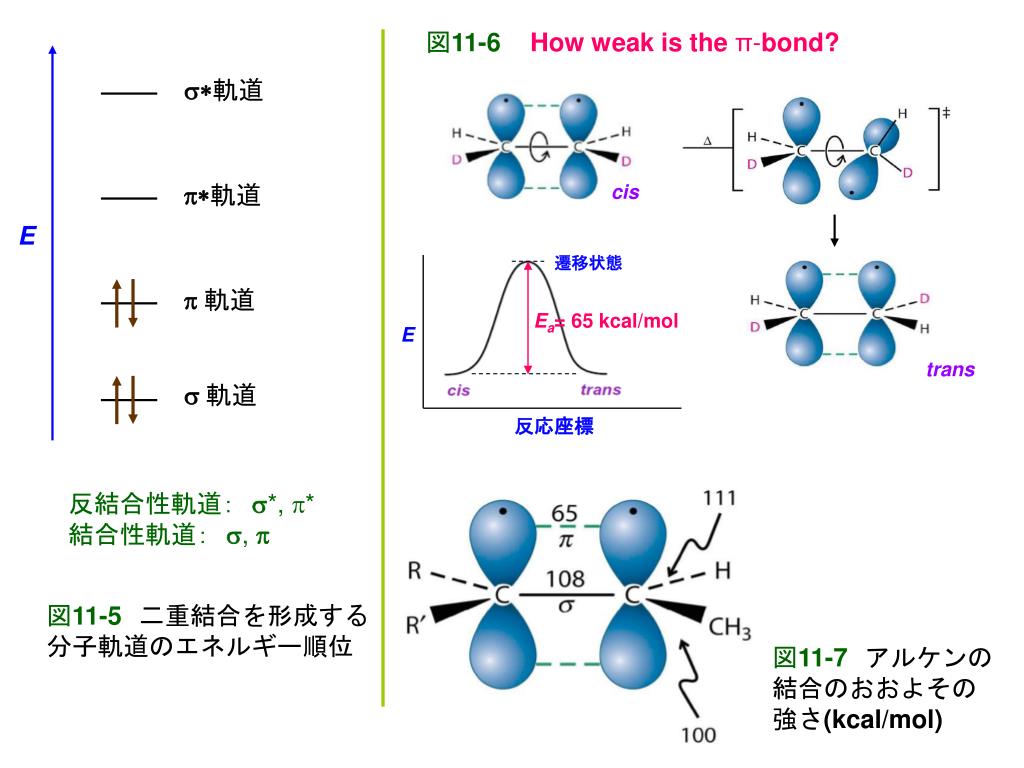
【ふるさと納税】 いろはす 北海道限定 ラベル 【 選べる 本数 お届け回数 】 540ml PET 24本 48本 定期便 単品 2ヶ月 3ヶ月 い・ろ・は・す 1箱 2箱 白旗山 ミネラルウォーター 飲料水 ペットボトル 常温 鉱水 水 天然水 飲料 防災 備蓄 高評価 北海道 札幌市
8,000円
23 customer ratings
4.91 ★★★★★
一滴一滴、森が育んだ、おいしい天然水『い・ろ・は・す天然水』から、北海道限定ラベルデザインが登場です。 北海道の大自然が育んだ天然水を、コカ・コーラ独自の厳しい品質管理を経て、みなさまにお届けします。…





![クリスタルガイザー 水( 500ml×48本入)【2shdrk】【クリスタルガイザー(Crystal Geyser)】[水 ミネラルウォーター 500ml カリフォルニア]](https://thumbnail.image.rakuten.co.jp/@0_mall/soukaidrink/cabinet/074/9000009984074.jpg?_ex=145x145)

![い・ろ・は・す ラベルレス(560ml*48本セット)【いろはす(I LOHAS)】[水 ミネラルウォーター]](https://thumbnail.image.rakuten.co.jp/@0_mall/soukaidrink/cabinet/174/82174.jpg?_ex=145x145)


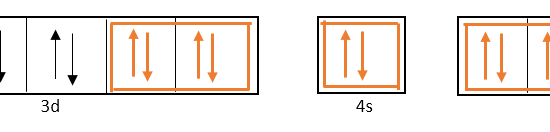


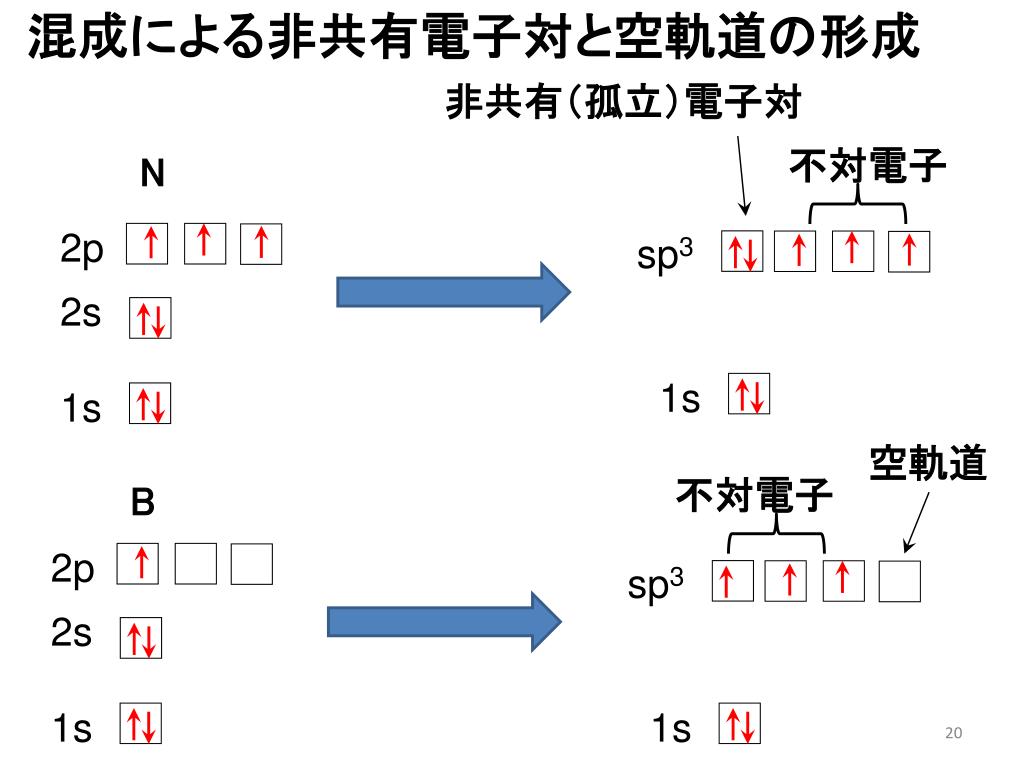











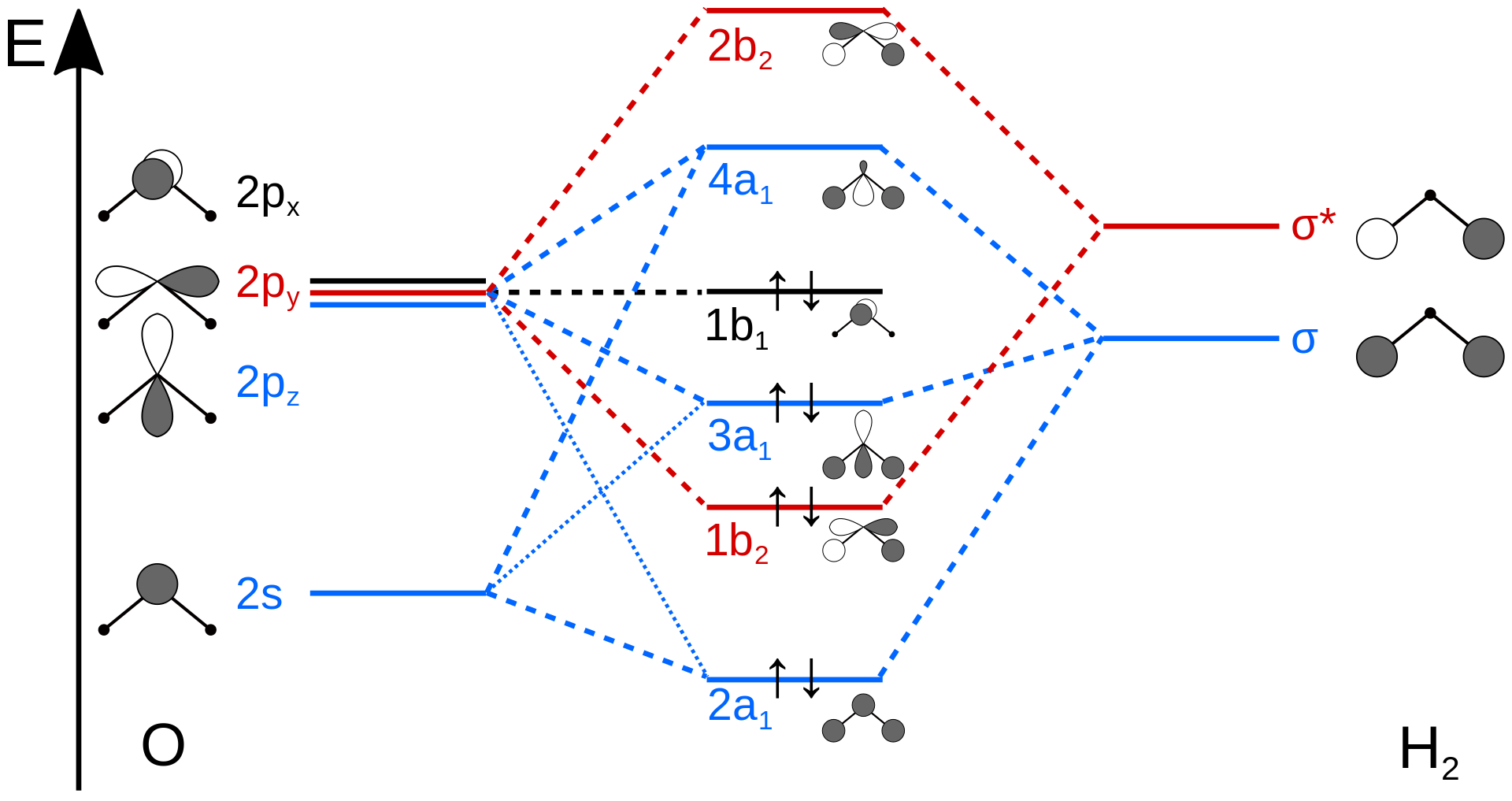
![化学新シリーズ 分子軌道法[POD版]Application of Molecular Orbital Theory to Organic Chemistry](https://www.shokabo.co.jp/sample/0646s.jpg)