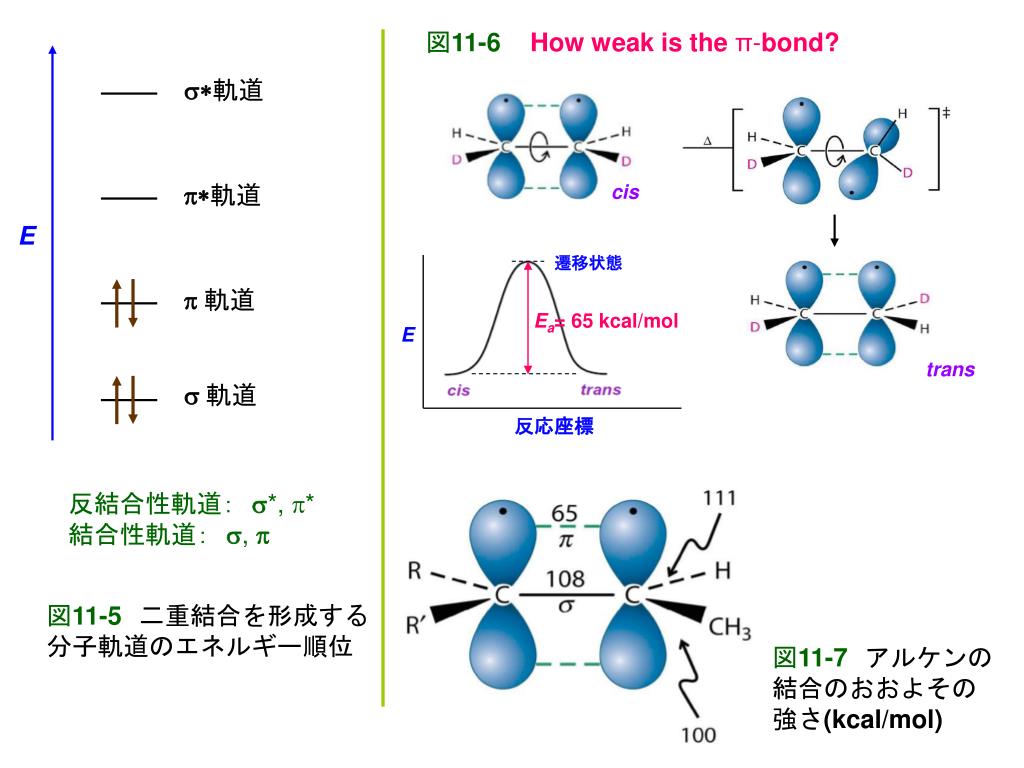
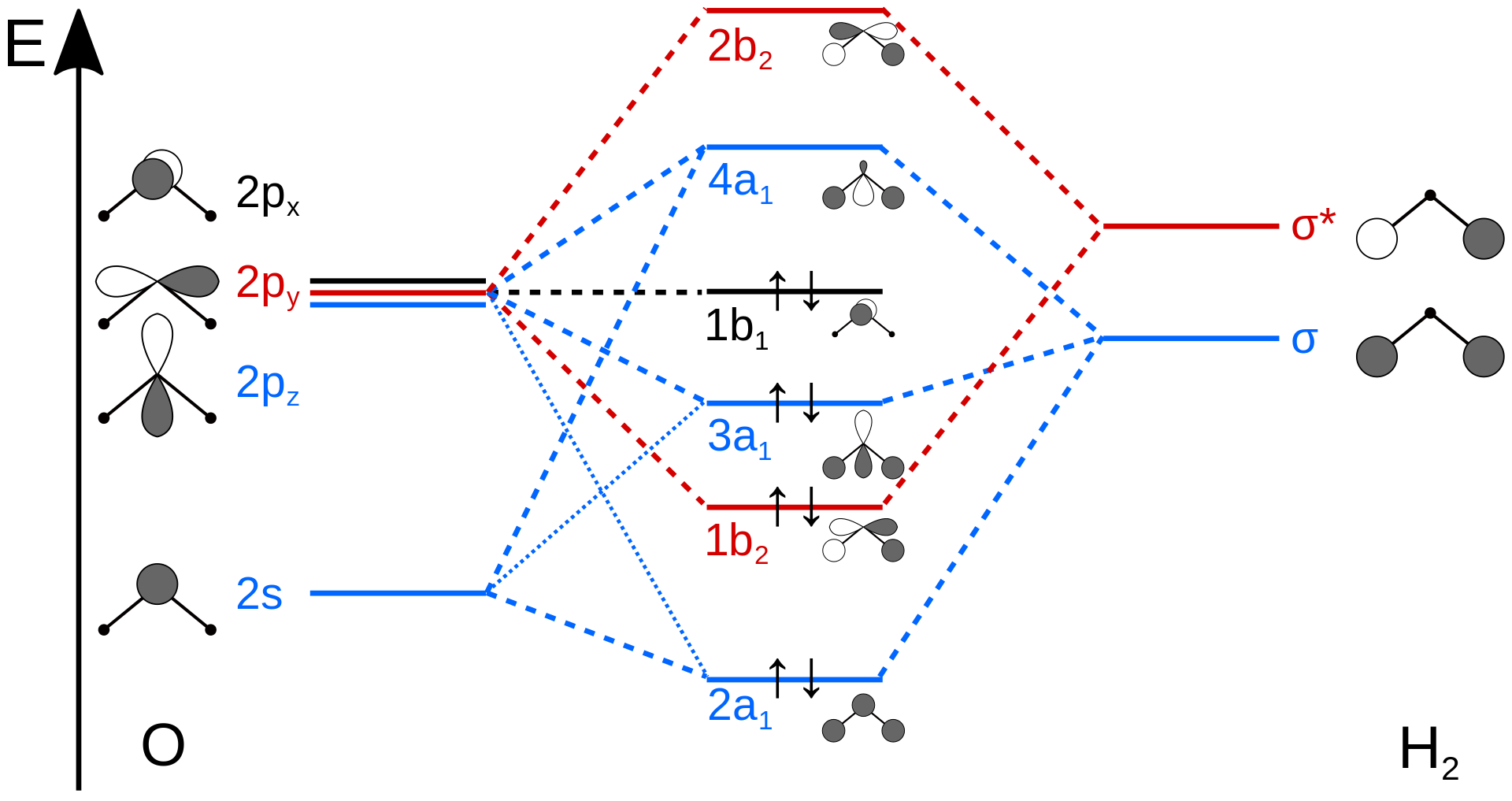
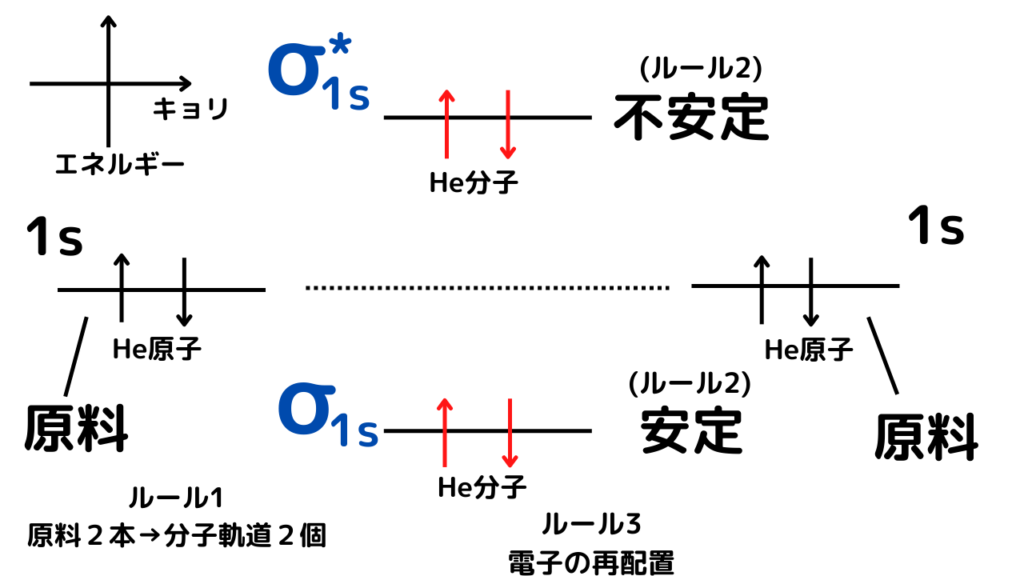
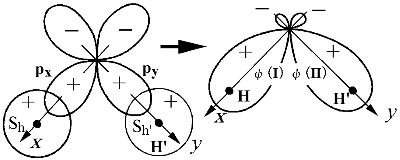
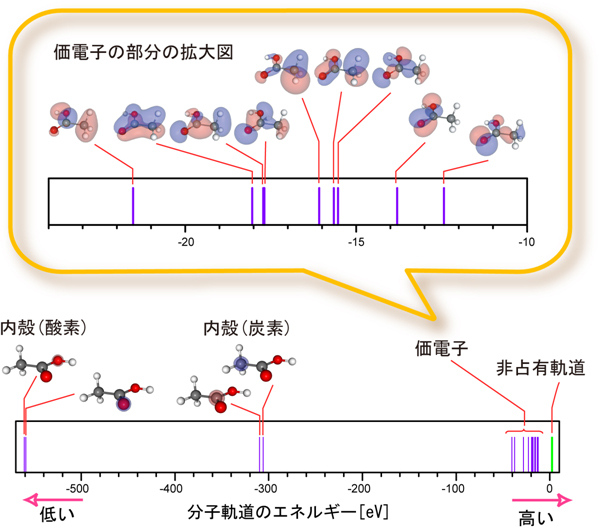
Images of 分子軌道
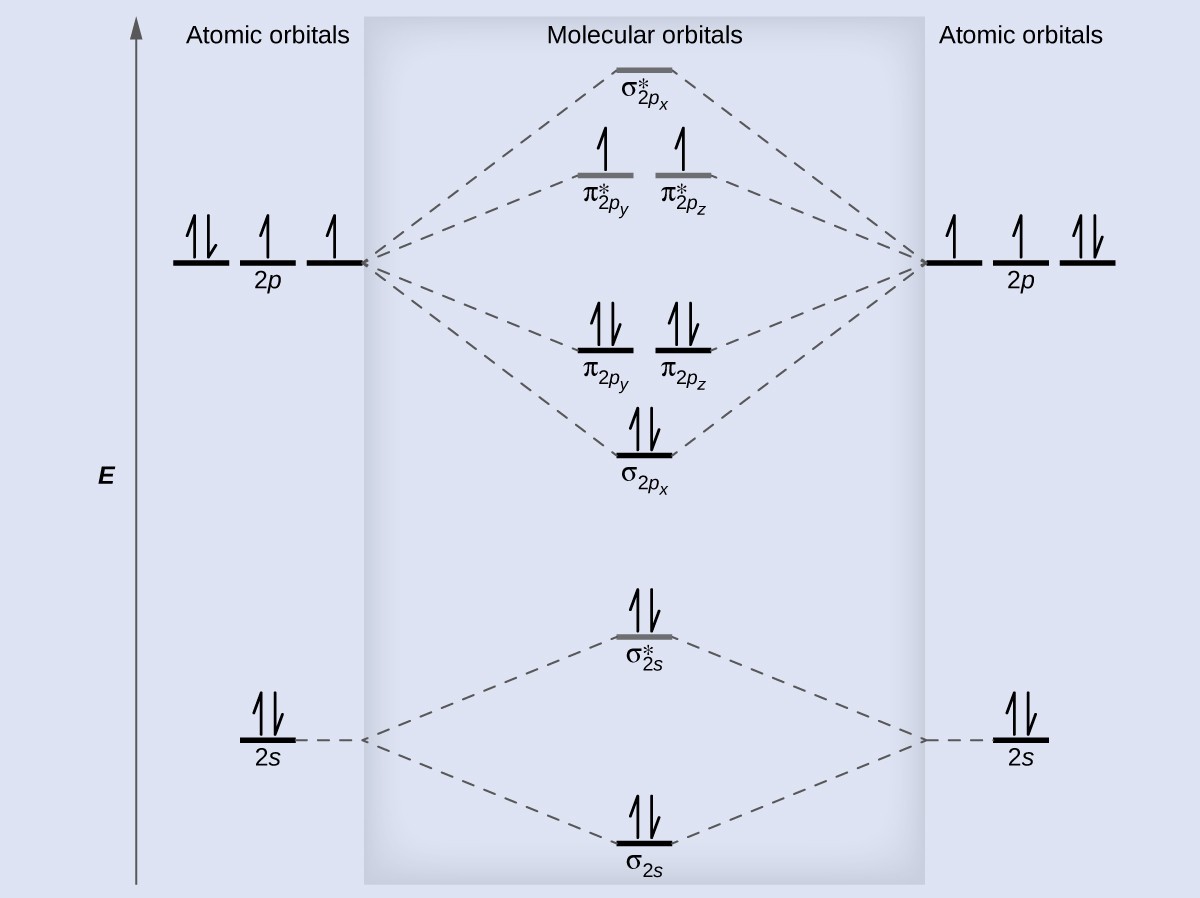
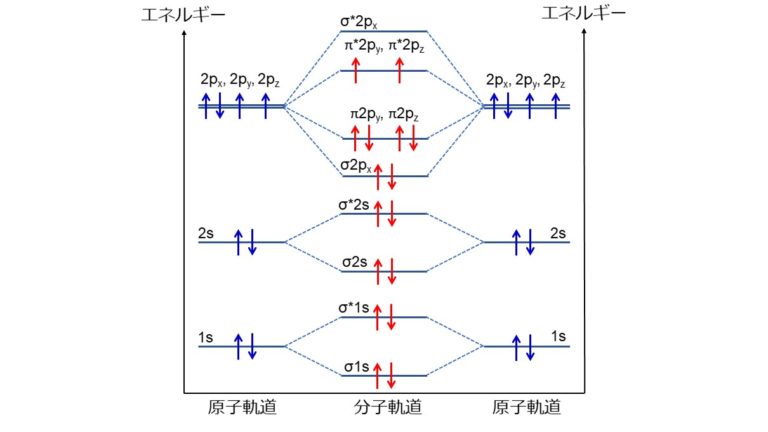
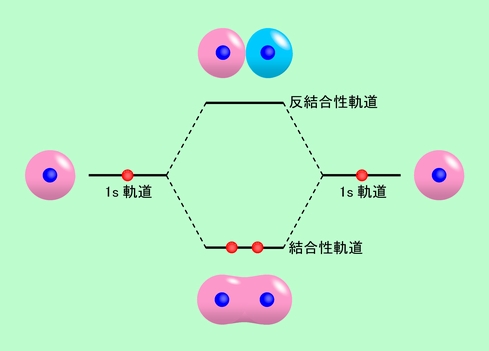
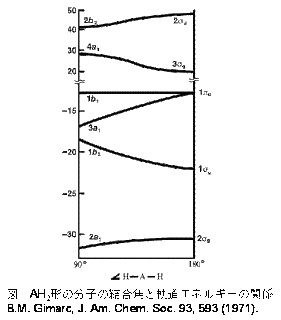
A diagram is shown that has an upward-facing vertical arrow running along the left side labeled, “E.” At the bottom center of the diagram is a horizontal line labeled, “sigma subscript 2 s,” that has two vertical half arrows drawn on it, one facing up and one facing down. This line is connected to the right and left by upward-facing, dotted lines to two more horizontal lines, each labeled, “2 s,” and with two vertical half arrows drawn on them, one facing up and one facing down. These two lines are connected by upward-facing dotted lines to another line in the center of the diagram, but farther up from the first and labeled, “sigma subscript 2 s superscript asterisk.” This horizontal line has two vertical half-arrow drawn on it, one facing up and one facing down. Moving further up the center of the diagram is a horizontal line labeled, “sigma subscript 2 p subscript x,” which lies below two horizontal lines, lying side-by-side, and labeled “pi subscript 2 p subscript y,” and “pi subscript 2 p subscript z.” Both the bottom and top lines are connected to the right and left by upward-facing, dotted lines to three more horizontal lines, each labeled, “2 p,” on either side. These sets of lines each hold three upward-facing and one downward-facing half-arrow. They are connected by upward-facing dotted lines to another single line and then pair of double lines in the center of the diagram, but farther up from the lower lines. They are labeled, “sigma subscript 2 p subscript x superscript asterisk,” “pi subscript 2 p subscript y superscript asterisk,” and “pi subscript 2 p subscript z superscript asterisk,” respectively. The lower of these two central, horizontal lines each contain one upward-facing half-arrow. The left and right sides of the diagram have headers that read, ”Atomic orbitals,” while the center header reads, “Molecular orbitals.”
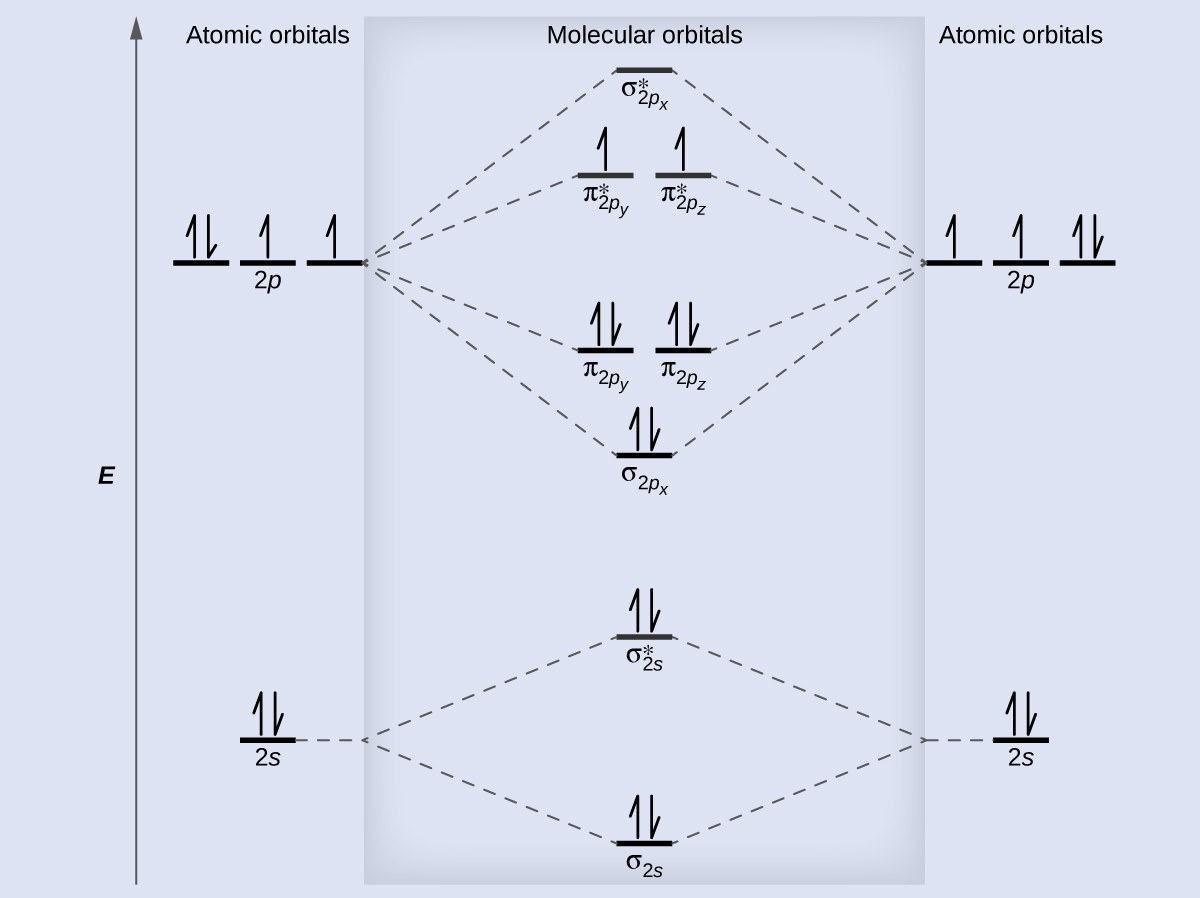
A diagram is shown that has an upward-facing vertical arrow running along the left side labeled, “E.” At the bottom center of the diagram is a horizontal line labeled, “sigma subscript 2 s,” that has two vertical half arrows drawn on it, one facing up and one facing down. This line is connected to the right and left by upward-facing, dotted lines to two more horizontal lines, each labeled, “2 s,” and with two vertical half arrows drawn on them, one facing up and one facing down. These two lines are connected by upward-facing dotted lines to another line in the center of the diagram, but farther up from the first and labeled, “sigma subscript 2 s superscript asterisk.” This horizontal line has two vertical half-arrow drawn on it, one facing up and one facing down. Moving further up the center of the diagram is a horizontal line labeled, “sigma subscript 2 p subscript x,” which lies below two horizontal lines, lying side-by-side, and labeled “pi subscript 2 p subscript y,” and “pi subscript 2 p subscript z.” Both the bottom and top lines are connected to the right and left by upward-facing, dotted lines to three more horizontal lines, each labeled, “2 p,” on either side. These sets of lines each hold three upward-facing and one downward-facing half-arrow. They are connected by upward-facing dotted lines to another single line and then pair of double lines in the center of the diagram, but farther up from the lower lines. They are labeled, “sigma subscript 2 p subscript x superscript asterisk,” “pi subscript 2 p subscript y superscript asterisk,” and “pi subscript 2 p subscript z superscript asterisk,” respectively. The lower of these two central, horizontal lines each contain one upward-facing half-arrow. The left and right sides of the diagram have headers that read, ”Atomic orbitals,” while the center header reads, “Molecular orbitals.”




![[200円OFFクーポン配布中] エクエル パウチ 120粒入り 大塚製薬 エクオール [ 正規品 送料無料 ] 4粒で S-エクオール 10mg 120粒 エクオール 大豆イソフラボン サプリ / EQUELLE エクエル大塚製薬 エクオル 最安値 挑戦中 [メール便]](https://thumbnail.image.rakuten.co.jp/@0_mall/pycno/cabinet/cmp/sale01/1231event-eq_05.jpg?_ex=300x300)



















![【先着順クーポン配付中】【2個セット】大塚製薬 エクエル パウチ 120粒入り×2個[エクエル 大塚製薬 エクオール 大豆 更年期 乳酸菌 健康 美容 サプリ]■(追跡付)メール便専用商品■正規取扱店ヤマト](https://thumbnail.image.rakuten.co.jp/@0_mall/shimin2/cabinet/shohin_otsuka/01eqll/eqll_389-02.jpg?_ex=300x300)

![化学新シリーズ 分子軌道法[POD版]Application of Molecular Orbital Theory to Organic Chemistry](https://www.shokabo.co.jp/sample/0646s.jpg)

![【ポイント10倍 1/9(金) 20:00〜1/16(金) 1:59まで】プレミアムカロリミット<機能性表示食品>【ファンケル 公式】 [FANCL ダイエットサプリ ダイエット サポート サプリメント キトサン カロリー サプリ 健康食品 桑の葉 くわのは サポニン 女性 男性 血中中性脂肪 ]](https://thumbnail.image.rakuten.co.jp/@0_mall/fancl-shop/cabinet/marathon/m_20260109/precalo_p10.jpg?_ex=300x300)